A newly published study on patients with primary and secondary liver cancer shows how Perspectum’s non-invasive MRI technology can help predict those at increased risk of poor surgical outcomes, potentially reducing healthcare costs by shortening length of hospital stay. The new technology, Hepatica, seamlessly integrates into routine pre-operative MRI assessment and is the only tool that combines pre-surgical measurements of liver health with AI-driven liver volumetry, providing actionable information to inform surgical decision-making and improve patient care.
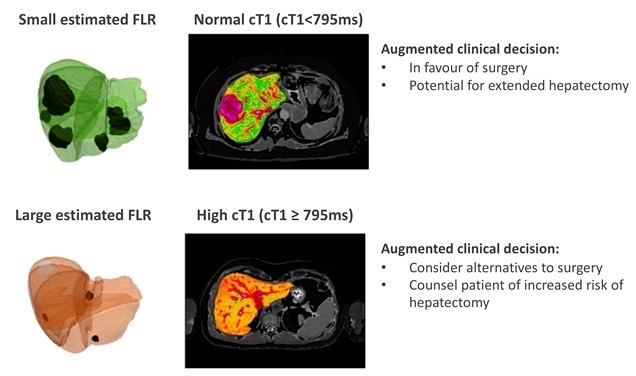
For liver cancer patients, post-operative outcomes are affected by the health of the liver tissue that will remain after surgery. Liver health can be compromised by chronic liver disease (CLD), which has a high and growing prevalence, with obesity and fatty liver disease on the rise. Patients with CLD are often asymptomatic until the point of decompensated cirrhosis, resulting in a large number of undiagnosed patients, especially those with secondary liver cancers. There is therefore an urgent need for tools that can help identify background liver disease to allow for better assessment of patient risk and postoperative outcomes. These unmet needs are addressed by Hepatica, a non-invasive MRI-based technology developed by Perspectum.
Hepatica helps surgeons to identify and characterize previously undiagnosed background liver disease, which can directly impact their clinical and surgical decision making. The non-invasive MRI-based tool combines LiverMultiScan® cT1 and PDFF, respective measures of liver fibroinflammation and fat, with future liver remnant (FLR) volume to evaluate an individual’s risk of having a poor post-operative outcome. In a recent study, LiverMultiScan was shown to be the best predictor of outcomes in chronic liver disease patients [Jayaswal et al, 2020]. In the current, prospective, observational study of 143 patients being considered for liver cancer surgery, elevated cT1 corresponded to a longer post-operative stay in hospital, with the combination of cT1 and FLR predicting poor liver performance in the 5 days immediately following surgery, and correlating with liver regenerative performance in the 3 months following surgery.
As surgeons, we are constantly trying to make operations safer and have improved outcomes for patients. Operating on the liver to remove cancer can give patients with advanced cancer a chance of a cure, and Hepatica allows us to plan that surgery with greater precision. Hepatica now allows us to make better informed decisions about the extent of safe surgery. This allows patients and the whole surgical team to approach the challenge with more information about their future liver performance.”
Professor Damian J. Mole, lead author on the study, Professor of Clinical and Experimental Surgery, Honorary Consultant Surgeon, University of Edinburgh
The results of this study, published in PLOS ONE, demonstrate the potential for Hepatica to transform the clinical decision-making process for liver cancer surgery, helping to provide personalized assessment of background liver disease, to better inform tumors boards and multidisciplinary teams, improve patient care, and save costs by reducing length of hospitalization.